UDI Update (2021)
The EU Medical Device Regulation deadlines have been updated as follows:
- May 2021 - Class III - high risk medical products e.g. cardiac catheters, heart valves etc.
- May 2023 - Class IIa - medium risk medical products, Class IIb - increased risk medical products.
- May 2025 - Class I - low risk medical products.
If you wish to discuss labelling, scanning and verifying your UDI code to ensure it meets the required standards, please contact us.
What is Unique Device Identification?
The FDA has established a unique device identification (UDI) system to identify and trace medical devices throughout their distribution and use. The UDI Final Rule was published 24th September 2013.
UDI is expected to improve greatly upon patient safety and healthcare industry processes by implementing a global system of standards which is fundamental to enabling traceability of medical devices across all healthcare stakeholders worldwide.
As part of this mandate, labels on medical devices must contain a unique device identifier (UDI) code in human- and machine- readable form. The machine-readable form must be able to be interpreted by automatic identification and data capture (AIDC) technology; that is, a barcode symbol or machine-readable text (OCR) that can be decoded by a barcode reader or machine vision system.
readable form. The machine-readable form must be able to be interpreted by automatic identification and data capture (AIDC) technology; that is, a barcode symbol or machine-readable text (OCR) that can be decoded by a barcode reader or machine vision system.
The FDA UDI rule will be rolled out in stages until September 2020, with deadlines to implement UDI labelling practices for a unique class of medical device at each stage. Medical device classes are defined by the FDA as Class I, Class II, and Class III to indicate lowest to highest risk should a device fail to operate.
Look up your medical device's class in the FDA's Product Classification Database.
What Is a UDI Code?
A UDI is a unique numeric or alphanumeric code consisting of two basic parts:
Device Identifier (DI) – A mandatory, fixed portion that identifies the device labeller and the specific version or model of a device. Every DI must be submitted to the GUDID (Global Unique Device Identification Database).
Production Identifier (PI) – A conditional, variable portion of a UDI. PIs are maintained by the labeller for internal operations and are not submitted to the GUDID.
All DI and PI elements of a UDI code must be properly formatted according to the requirements of a labeller's UDI issuing agency (IA) (an FDA-accredited organisation that assigns a UDI to a labeller) in both human- and machine-readable format in order to be considered UDI compliant.
How Do I Know if My Barcodes Are UDI Compliant?
Verification is the measurement of the quality of a 1D barcode or 2D symbol according to an agreed-upon  quality standard. Microscan Barcode Verification Systems are fully-integrated off-line solutions for label testing, programmed to assess the accuracy of data encoded in 1D/2D codes against data structure specifications regulated by UDI issuing agencies (such as GS1 and HIBCC) as well as barcode print quality standards (such as ISO/IEC).
quality standard. Microscan Barcode Verification Systems are fully-integrated off-line solutions for label testing, programmed to assess the accuracy of data encoded in 1D/2D codes against data structure specifications regulated by UDI issuing agencies (such as GS1 and HIBCC) as well as barcode print quality standards (such as ISO/IEC).
Microscan LVS Barcode Verifiers with LVS-95XX Software automatically identify barcode types and apply 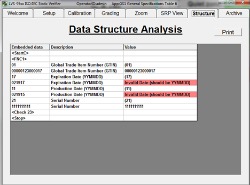 the appropriate verification parameters for each symbol according to the user's selected GS1 or HIBCC specifications for their device. If there is an error in the format of a UDI code, the LVS-95XX Software will highlight the error for easy diagnostics.
the appropriate verification parameters for each symbol according to the user's selected GS1 or HIBCC specifications for their device. If there is an error in the format of a UDI code, the LVS-95XX Software will highlight the error for easy diagnostics.
Want to learn more?
Learn the Basics of UDI:
What are Class I, Class II, and Class III devices?
Where do I get a Device Identifier (DI)?
What is the timeline to become UDI compliant?
Contact us to receive a copy of the Microscan UDI Compliance Guide.
Source: http://www.microscan.com/en-us/Technology/Verification/FDA-UDI-Rule.aspx